On presentation his B.P = 125/80mmHg, Pulse = 85 bpm , Afebrile, R.R = 15/min, Spo2 = 99% at room air. Chest was Clear, audible S1, S2 without any added sound or murmur , JVP wasn’t raised, no pedal edema
Baseline Labs:
◦Na = 138 mEq/L
◦K= 4.1 mEq/L
◦Mg = 2.1 mg/dL
◦Ca = 9.4 mg/dL
◦Cr= 0.9 mg/dL
◦CBC – Normal
◦Cardiac Enzymes - Normal
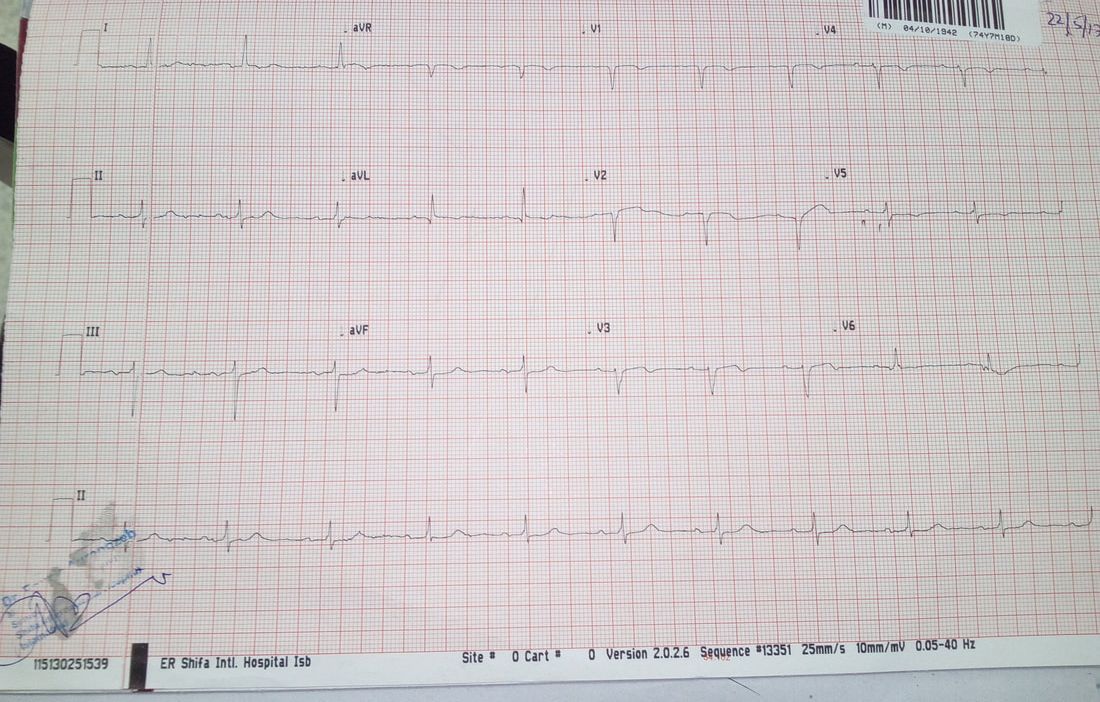
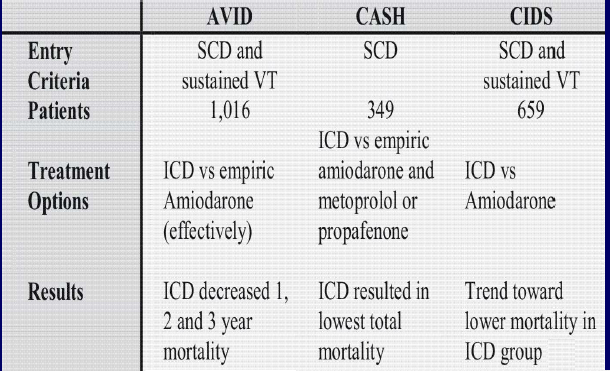
ECG
◦evidence of old anterior wall MI
◦No arrhythmia detected at presentation
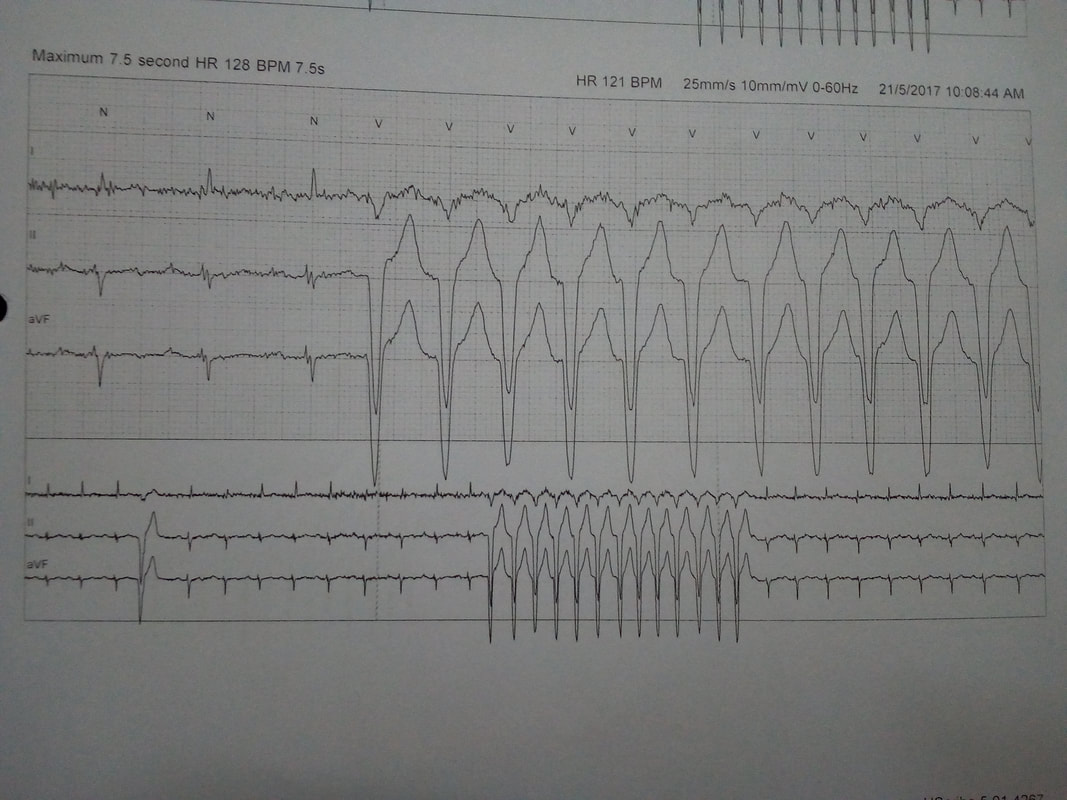
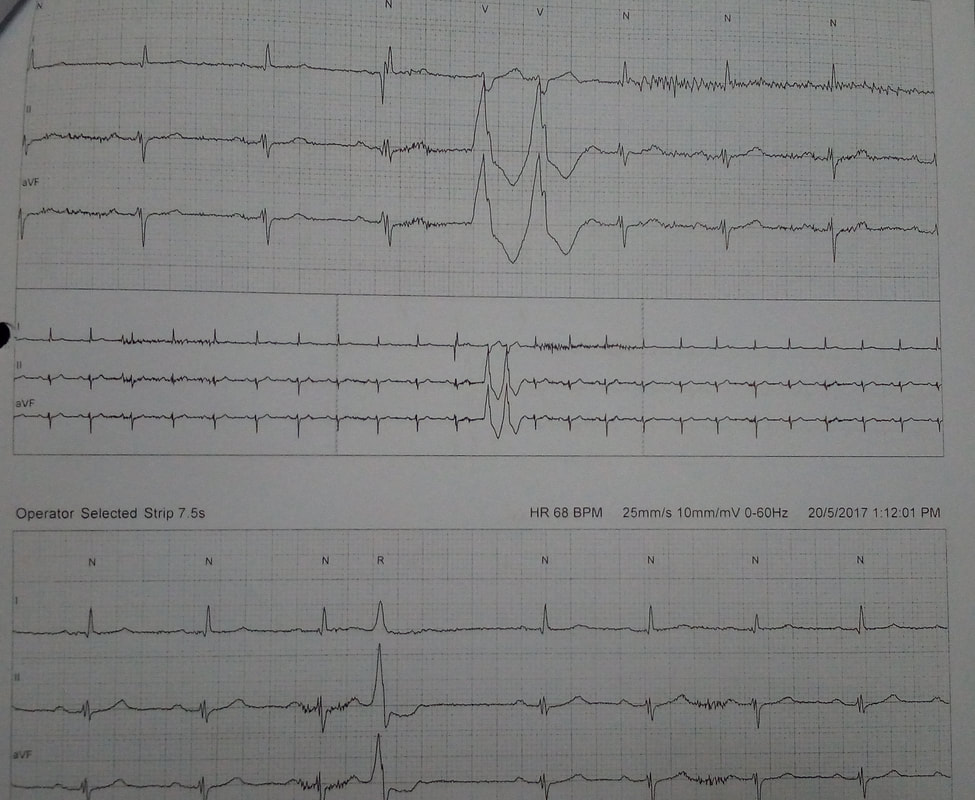
◦Multiple PVCs
◦Runs of sustained VT



 RSS Feed
RSS Feed





